40 phase labels in chemical equations
End-of-Chapter Material | Introductory Chemistry Chemical equations can also be used to represent physical processes. Write a chemical reaction for the freezing of water, including the proper phase labels. Explain why 4 Na (s) + 2 Cl2(g) → 4 NaCl (s) should not be considered a proper chemical equation. Explain why H2(g) + 1/2 O2(g) → H2O (ℓ) should not be considered a proper chemical equation. PDF Chapter 8 chemical equations and reactions test answer key Chemical equations can also be used to represent physical processes. Write a chemical reaction to boiling water, including the appropriate phase labels. Chemical equations can also be used to represent physical processes. Write a chemical reaction to freezing water, including the appropriate phase labels. Explain why 4NA (s) + Ã, 2cl2 (g) Ã, Ã,
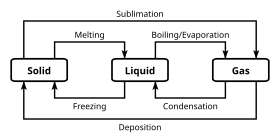
Chapter 5 - Chemical Reactions and Equations - CHE 105/110 ... Many chemical equations also include phase labels for the substances: (s) for solid, (ℓ) for liquid, (g) for gas, and (aq) for aqueous (i.e., dissolved in water). Special conditions, such as temperature, may also be listed above the arrow. For example, 2NaHCO3 (s) − →−200°C Na2CO3 (s) +CO2(g) +H2O(ℓ) Key Takeaways
Phase labels in chemical equations
Chapter 7 So lets write the ionic and net ionic equations for the two equations above. The first equation we'll convert is; Na 2 SO 4(aq) + CaCl 2(aq)---> CaSO 4(s) + 2NaCl (aq) To change the above equation to an ionic equation the aqueous ionic substances must be written as ions and any solid, liquid or gas remains in its molecular form. State symbols and phase changes | StudyPug The phase can affect how reactive a substance is, but changing phase (a physical change) is not the same as changing the substance (a chemical change). A fully detailed chemical equation will show the state (or phase) of matter that the atoms or molecules are in. These states are: Solid, given the symbol (s) Liquid, given the symbol (l) The Chemical Equation - Introductory Chemistry - 1st ... Write and balance the chemical equation that represents nitrogen and hydrogen reacting to produce ammonia, NH 3. Answer N 2 + 3H 2 → 2NH 3 Many chemical equations also include phase labels for the substances: (s) for solid, (ℓ) for liquid, (g) for gas, and (aq) for aqueous (i.e., dissolved in water).
Phase labels in chemical equations. 5.7 End-of-Chapter Material - Introductory Chemistry - 1st ... Chemical equations can also be used to represent physical processes. Write a chemical reaction for the freezing of water, including the proper phase labels. Explain why 4 Na (s) + 2 Cl2(g) → 4 NaCl (s) should not be considered a proper chemical equation. Explain why H2(g) + 1/2 O2(g) → H2O (ℓ) should not be considered a proper chemical equation. End-of-Chapter Material - Introductory Chemistry - 1st ... Chemical equations can also be used to represent physical processes. Write a chemical reaction for the freezing of water, including the proper phase labels. Explain why 4Na (s) + 2Cl 2 (g) → 4NaCl (s) should not be considered a proper chemical equation. Explain why H 2 (g) + ½O 2 (g) → H 2 O (ℓ) should not be considered a proper chemical equation. Solved Pre-lab Questions: 1. Write a balanced chemical ... Write a balanced chemical equation including phase labels for the reaction between aqueous copper (II) nitrate and aqueous sodium hydroxide 2. Nitrogen monoxide (NO) and nitrogen dioxide (NO2) are toxic, corrosive gases that significantly lower blood pressure when inhaled. How are these gases produced in today's experiment? What should 7.3: The Chemical Equation - Chemistry LibreTexts In the symbolic equation, chemical formulas are used instead of chemical names for reactants and products, while symbols are used to indicate the phase of each substance. It should be apparent that the chemical shorthand method is the quickest and clearest method for writing chemical equations.
RLC Circuit Equations & Example | What is a RLC Circuit ... Mar 14, 2022 · RLC Circuit Equations. Recall that in an AC circuit of resistor alone, the voltage across a resistor is given by the formula of Ohm's law: {eq}V_R=I*R {/eq} where phasor {eq}V_R {/eq} is in phase ... Phase Definition and Examples - ThoughtCo A phase of matter is characterized by having relatively uniform chemical and physical properties. Phases are different from states of matter. The states of matter (e.g., liquid, solid, gas) are phases, but matter can exist in different phases yet remain in the same state of matter. For example, liquid mixtures can exist in multiple phases, such ... Symbols in Chemical Equations - Harper College Symbols in Chemical Equations. Symbol: Meaning + used to separate one reactant or product from another used to separate the reactants from the products - it is pronounced "yields" or "produces" when the equation is read used when the reaction can proceed in both directions - this is called an equilibrium arrow and will be used later in the ... pycse - Python3 Computations in Science and Engineering Note that only one root is real (and even then, we have to interpret 0.j as not being imaginary. Also, in a cubic polynomial, there can only be two imaginary roots). In this case that means there is only one phase present.
5.2 Chemical Equations | The Basics of General, Organic ... It is not uncommon to include a phase label with each formula— (s) for solid, (ℓ) for liquid, (g) for gas, and (aq) for a substance dissolved in water, also known as an aqueous solution. If we included phase labels for the reactants and products, under normal environmental conditions, the reaction would be as follows: H2(g) + O2(g) → H2O (ℓ) Note Phase Rule - Teaching Phase Equilibria The system is entirely composed of H 2 O, so there is only one component present.; The phases present represent three states of matter: liquid (water), solid (ice), and vapor (steam). All have distinct physical properties (e.g. density, structure--or lack of, etc.) and chemical properties (e.g. Δ G formation, molar volume etc.) so they must be considered distinct phases. Chapter 4 Quantities of reactants and products 3 Steps for Balancing Chemical Equations - ThoughtCo To do this, you need to be familiar with the properties of various compounds or you need to be told what the phases are for the chemicals in the reaction. Oxides are solids, hydrogen forms a diatomic gas, tin is a solid, and the term ' water vapor ' indicates that water is in the gas phase: SnO 2 (s) + 2 H 2 (g) → Sn (s) + 2 H 2 O (g)
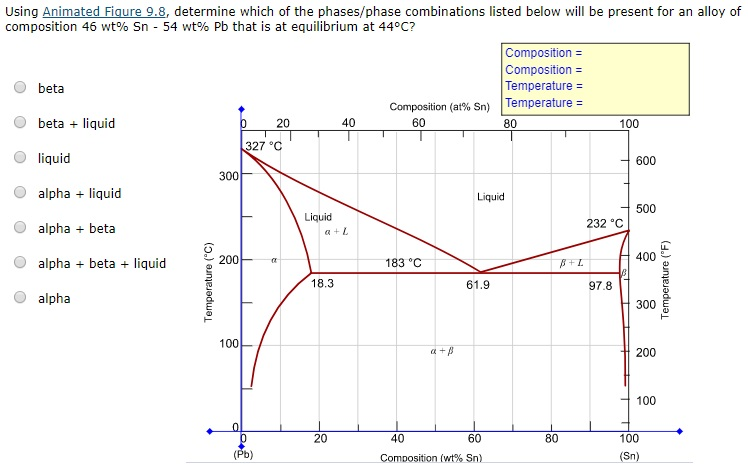
Phase Diagrams - Chemistry Using the phase diagram for water given in [link], determine the state of water at the following temperatures and pressures: (a) −10 °C and 50 kPa (b) 25 °C and 90 kPa (c) 50 °C and 40 kPa (d) 80 °C and 5 kPa (e) −10 °C and 0.3 kPa (f) 50 °C and 0.3 kPa Solution
A thermochemical equation is a balanced chemical reaction ... A thermochemical equation is a balanced chemical reaction equation (including phase labels) with the enthalpy of reaction value written directly after the equation THERMOCHEMICAL EQUATIONS A reaction that produces heat is referred to as exothermic. For exothermic reactions, enthalpy values are assigned a negative value (-DH).
Design equations of Rectangular Microstrip Patch Antenna May 17, 2020 · Chemical Waste: A. Discarded disinfectants, Cleaning agents B. X-ray film developing liquid, Infected secretions C. Aspirated body fluids, Liquid from laboratories 6. Microbiology, Biotechnology, and other clinical lab waste 7. Chemical Liquid Waste Yellow bin/ bag with the cytotoxic label: 1.
Ordinary Differential Equations · DifferentialEquations.jl AutoVern7(Rodas5()) handles both stiff and non-stiff equations in a way that's efficient for high accuracy. Tsit5() for standard non-stiff. This is the first algorithm to try in most cases. BS3() for fast low accuracy non-stiff. Vern7() for high accuracy non-stiff. Rodas4() or Rodas5() for small stiff equations with Julia-defined types, events ...
Phase diagram - Wikipedia A phase diagram in physical chemistry, engineering, mineralogy, and materials science is a type of chart used to show conditions (pressure, temperature, volume, etc.) at which thermodynamically distinct phases (such as solid, liquid or gaseous states) occur and coexist at equilibrium . Contents 1 Overview 2 Types 2.1 2-dimensional diagrams
What are Chemical Equations? Detailed Explanation, Examples The reactants and the products (for which the chemical formulae are written in chemical equations) can be separated by one of the following four symbols. In order to describe a net forward reaction, the symbol '→' is used. In order to describe a state of chemical equilibrium, the symbol '⇌' is used.
chemical equation - Yeah Chemistry Formulas of the reactants appear on the left side of the equation; those of products are written on the right. In many cases it is useful to indicate the state or phases of the substance in an equation.You use the following phase labels: (g) = gas , (l) = liquid , (s) = solid , (aq) = water solution
PDF Chemical reaction : conversion of substances into ... Chemical reaction : conversion of substances into different substances (by rearranging atoms) Reactants: substances present before reaction Products: substances present after reaction Chemical equation : represents a reaction on paper Reactants →Products Phase labels : show the phase of reactants or products (s) : (l) : (g) : (aq ) :
Guide for authors - Chemical Engineering Journal - ISSN 1385-8947 Theophilos Ioannides: Electrochemical energy storage (batteries, supercapacitors), Dielectric capacitors, Thermal energy storage (phase change materials), Chemical energy storage, Solar evaporation, Thermoelectrics, Electromagnetic wave absorption and interference shielding, Triboelectric generators, Energetic materials Reviews and Perspectives:
PDF Strategy to Determine the Phase of a Chemical Mixture Ph or phase nc is the number of components Pure-Component Mixture (nc= 1, a pure compound ) For P= 1 atm, find T mand T bin Table B.1 of F&R, 3rdEd. If T > T b, then Ph= vapor where is T b the boiling point If T = T b, then Ph= vapor-liquid If T m< T < T
Chemical Equations - GitHub Pages It is not uncommon to include a phase label with each formula—(s) for solid, (ℓ) for liquid, (g) for gas, and (aq) for a substance dissolved in water, also known as an aqueous solution. If we included phase labels for the reactants and products, under normal environmental conditions, the reaction would be as follows: H 2 (g) + O 2 (g) → H ...
End-of-Chapter Material - GitHub Pages Chemical equations can also be used to represent physical processes. Write a chemical reaction for the freezing of water, including the proper phase labels. Explain why 4Na (s) + 2Cl2(g) → 4NaCl (s) should not be considered a proper chemical equation. Explain why H2(g) + 1/2O2(g) → H2O (ℓ) should not be considered a proper chemical equation.
Solved Phase labels never appear in balanced chemical ... Question: Phase labels never appear in balanced chemical equations true or false ? This problem has been solved! See the answer See the answer See the answer done loading
5.1 The Chemical Equation - Introductory Chemistry - 1st ... Many chemical equations also include phase labels for the substances: (s) for solid, (ℓ) for liquid, (g) for gas, and (aq) for aqueous (i.e., dissolved in water). Special conditions, such as temperature, may also be listed above the arrow. For example, 2 NaHCO 3 (s) →200°C Na 2 CO 3 (s) + CO 2 (g) + H 2 O(ℓ)
The Energy in Chemical Reactions: Thermodynamics and Enthalpy During a chemical reaction, old bonds between atoms break and new bonds form. Breaking chemical bonds requires enthalpy; it is an endothermic process. Forming new bonds releases enthalpy; it is exothermic. When a chemical reaction occurs, the balance between these two processes determines if the reaction is endothermic or exothermic overall. A ...
Chapter 4 Section E Neutralization Reactions Write a balanced chemical equation for each neutralization reaction in Exercise 4. 3HBr +Fe(OH) 3 --> FeBr 3 +3H 2 O 3HNO 2 +Al(OH) 3 --> Al(NO 2 ) 3 +3H 2 O 2HClO 3 +Mg(OH) 2 --> Mg(ClO 3 ) 2 +2H 2 O 7. Write a balanced chemical equation for the neutralization reaction between each given acid and base. Include the proper phase labels.
Math Principles This website will show the principles of solving Math problems in Arithmetic, Algebra, Plane Geometry, Solid Geometry, Analytic Geometry, Trigonometry, Differential Calculus, Integral Calculus, Statistics, Differential Equations, Physics, Mechanics, Strength of Materials, and Chemical Engineering Math that we are using anywhere in everyday life.
The Chemical Equation - Introductory Chemistry - 1st ... Write and balance the chemical equation that represents nitrogen and hydrogen reacting to produce ammonia, NH 3. Answer N 2 + 3H 2 → 2NH 3 Many chemical equations also include phase labels for the substances: (s) for solid, (ℓ) for liquid, (g) for gas, and (aq) for aqueous (i.e., dissolved in water).












Post a Comment for "40 phase labels in chemical equations"